Card Study
Providing cross-sectional descriptive data about clinical care, knowledge and behavior, perception of care, and prevalence of conditions.
In collaboration with:
WHAT IS A CARD STUDY?
A card study is a field-tested method for gathering data in the location where patients receive care by those who provide the care.
The card study method was pioneered by the Ambulatory Sentinel Practice Network (ASPN), and the method has been expanded by other practice-based research networks. An IRB protocol for streamlining human subject protection for multiple card studies in a network has been developed.
PROVEN METHOD USING TODAY’S TECHNOLOGY
Observational studies that collect patient-level survey data at the point-of-care. Card studies have been used to describe clinical problems, management, and outcomes in primary care for more than 40 years.
LOW BURDEN FOR BOTH CLINICIAN AND PRACTICE
Card studies are low-burden for a clinician and practice. The pocket-sized card was designed to take fewer than 60 seconds and allowed clinicians to carry it from room to room as they saw eligible patients.
ACTIONABLE DATA SHAPING FUTURE CARE
Card studies provide cross-sectional descriptive data about clinical care, knowledge and behavior, perception of care, and prevalence of conditions that may not be captured in the electronic health record.
FOR RESEARCHERS
Step 1
Create Your Card Study
Your clinicians will receive the invite to participate in the Card Study. They’ll want to know the purpose of your study as well as the completion criteria.
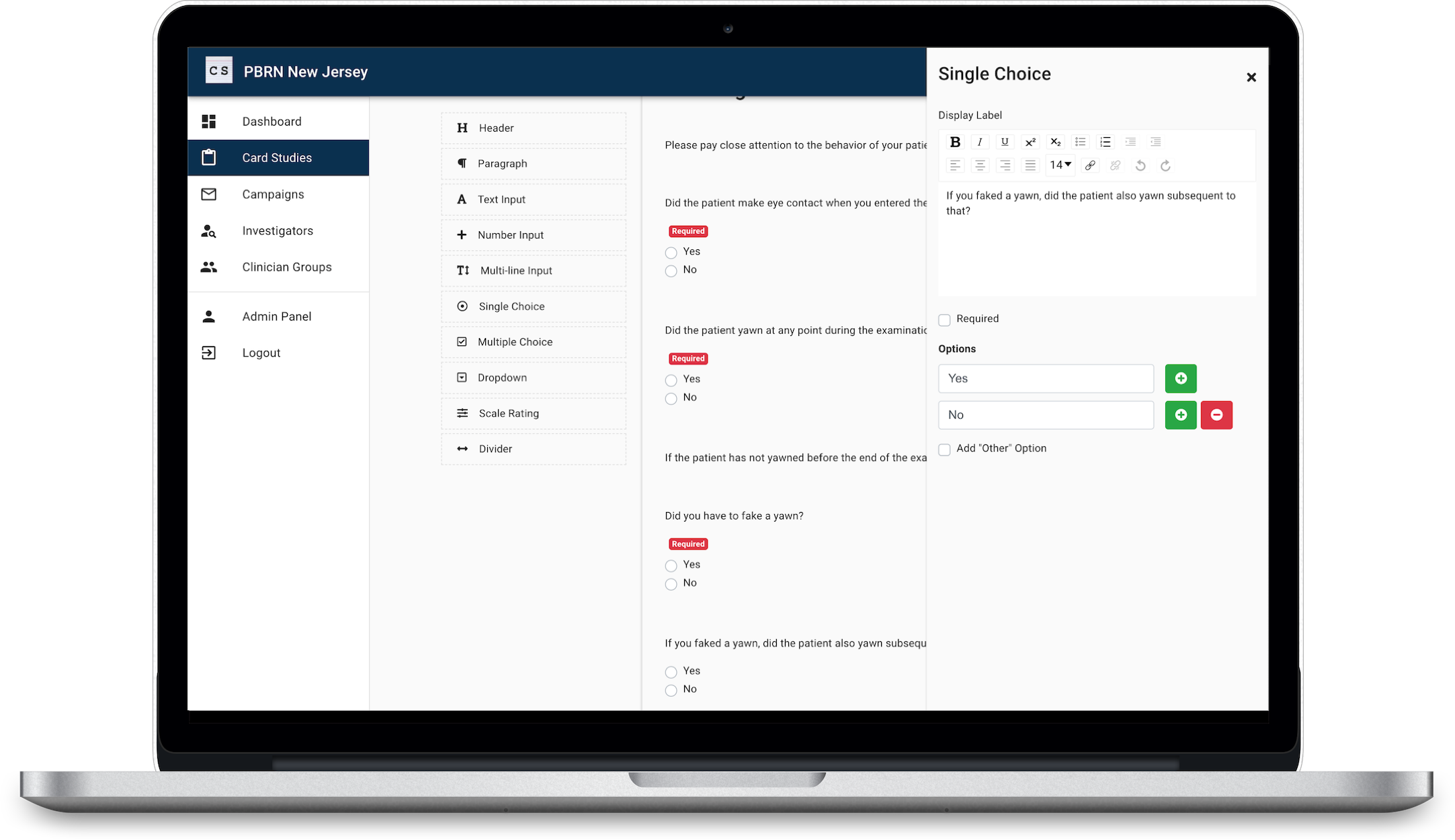
Use our survey builder tool to create your card. Common form elements available are Dropdown, Single Choice, Multiple Choice, Scale Rating, Number Input, Text Input, and many other options.
Once last check to review your Card. If you’re collaborating with other researchers, now is a great time to share your draft before creating a campaign.
Step 2
Create and Send Campaign
Here is where you will create your campaign that sends your Card Study to the clinicians. You can customize the invitation text, add tags to help classify your audience, and select automated reminder options.
You have the ability to enter individual emails manually, or bulk upload a file containing your clinician’s emails. You also can go back and edit your campaign if needed. When you’re all set, click SEND!
Step 3
Monitor Results in Realtime
You can monitor your campaign results in realtime, as your clinicians accept invitations and begin completing cards.
At any point, you can download response data from any or all campaigns for a given card.
FOR CLINICIANS
Accept an Invite
Accept or Decline invitations from researchers to participate in a study.
Join a Study
Submit responses until you meet the completion criteria for the card study.
Complete the Study
Once you have completed the requested number of submissions, you’re all set!